Over 3,000+ companies globally trust us
From startups to global enterprise giants, Certvalue empowers to extend the breadth and depth of their customer relationships








Get Instant Quote
Certvalue is one of the leading GMP Consultants in Burkina Faso for providing GMP certification in Burkina Faso, Ouagadougou, Bobo-Dioulasso, Banfora, Arbinda, Pouytenga, Ouahigouya and other major cities in Burkina Faso with the services of implementation, Documentation, Audit, Templates, Training, Gap Analysis, Registration at affordable cost to all organization to get Certified under Good Manufacturing Practice Management System in Burkina Faso .

Professional Consulting Company
CONSULTATION & CERTIFICATION
GMP Certification in Burkina Faso is used for good Manufacturing Practice (GMP) is a term that is recognized Globally for the control and management of manufacturing, testing the overall quality control of food and pharmaceutical products. GMP addresses a subject issue including documentation, recording information, qualifications, sanitation, cleanliness, equipment verification, validation process, and complaint handling. All most all GMP requirements are very general and open-ended, allowing each manufacturer to decide the best necessary controls. the quality approach of GMP Consulting Services in Burkina Faso secures manufacturing, enabling companies to minimize contamination, mistakes, and errors. This, in turn, protects the customer from purchasing a product, which is not effective or dangerous.
GMP registration in Burkina Faso is a system to make sure that products are always produced and controlled according to quality standards. It is designed to minimize the risks complex in any pharmaceutical production that cannot be eliminated through testing the final product.
Requirements of GMP Certification in Burkina Faso:
- Good documentation makes up a necessary part of the quality assurance system. Written policy prevents errors resulting from spoken communication, and clear documentation authorize tracing of activities performed.
- form must be designed, prepared, reviewed, and distributed with care.
- Documents must be approved, signed, and dated by the suitable competent and authorized persons.
- Documents must have distinct contents. The title, nature, and purpose should be distinctly stated. They must be laid out in a well-ordered fashion and be easy to check the reproduced documents must be clear and legible.
- Documents must be regularly reviewed and kept updated. When a document has been revised, systems must be operated to prevent accidental use of superseded documents (Example only current documentation should be accessible for use).
- Documents must not be handwritten; however, documents require the entry of data, these entries may be made in understandable legible handwriting using a suitable indelible medium (i.e., not a pencil). Enough space must be provided for such entries.
The following GMP Certification in Ouagadougou approach concern ‘documentation and records’ may be helpful for pharmaceutical manufacturers to meet the assumption of different regulatory organizations.
Write good procedures and follow them, think what has happened in a workspace if written procedures are not available. People depend on more senior employees to tell them how to do things and then do their job. This is exceptional for an organization making garden pots, but not so good when the products being made are pharmaceuticals and can even cause death! Figure the task before you begin writing the procedure. Create a brief crash of the important steps and key points related to the task.
Following procedures, it is all very well to have a great written plan of action in place but to ensure a controlled and compatible performance they need to be followed; it is a GMP requirement. Often, the steps described in a written policy may not be appear to the most efficient way of working, and taking shortcuts may save time.
These are the two main reasons for this:
- Many shortcuts may create a risk that can be expensive in the end.
- Each step in a procedure has been covered for a purpose.
Keep good records, Good records allow one to track all activities performed during batch manufacture, from the getting of raw materials to the final product release; they come up with a history of the batch and its distribution.
Benefits of using GMP Certification Services in Burkina Faso?
Benefits for the manufacturer one of the main benefits is remarkably improved quality systems and quality agreement at the excipient manufacturer. We have seen these improvements in the months leading up to GMP certification and carry on with during the years immediately following GMP certification. Some excipient mass producers were already at a high level of GMP compliance and certification.
Benefits of GMP Certification Services in Burkina Faso
GMP Services in Burkina Faso Enhances the food safety management system. GMP Consultants in Burkina Faso Increases Clients trust in your products
GMP Certification Cost in Burkina Faso Helps to decrease operating costs due to rework and penalties due to non-compliance
GMP in Burkina Faso Helps boost export opportunities.
Reduced duplication of inspections
Which industries eligible for GMP Certification in Burkina Faso?
- Manufacturing Companies
- Pharmaceuticals Companies
- Food Manufacturing Companies.
How to get GMP Consulting Services in Burkina Faso?
Certvalue is providing GMP Consulting Services in Burkina Faso with extensive expertise and experience in all International food and pharmaceutical products Standards. For GMP Certification in your organization, reach Certvalue – GMP Consultants us at +776017362326 Send your requirements to Our experts will call you and guide for Successful GMP Certification in Burkina Faso. We would be happy to assist your company in the GMP Certification process in Burkina Faso to send your research after contact @certvalue.com.
Quick Enquiry Form
Our Services
- ISO 9001 Certification
- ISO 14001 Certification
- OHSAS 18001 Certification
- ISO 22000 Certification
- ISO 27001 Certification
- ISO 13485 Certification
- ISO 17025 Certification
- ISO 45001 Certification
- ISO 22301 Certification
- ISO 27701 Certification
- ISO 20000-1 Certification
- ISO 27017 Certification
- ISO 27018 Certification
- ISO 50001 Certification
- ISO 55001 Certification
- ISO 21001 Certification
- ISO 28000 Certification
- ISO 27032 Certification
- ISO 15189 Certification
- ISO 22716 Certification
- ISO 22483 Certification
- CE mark Certification
- HACCP Certification
- HALAL Certification
- SA 8000 Certification
- ROHS Certification
- BIFMA Certification
- GMP Certification

Asked Any Questions
The certificate is issued from a premium certification body and it can be verified from its -website. Every certificate comes with a unique certification number.
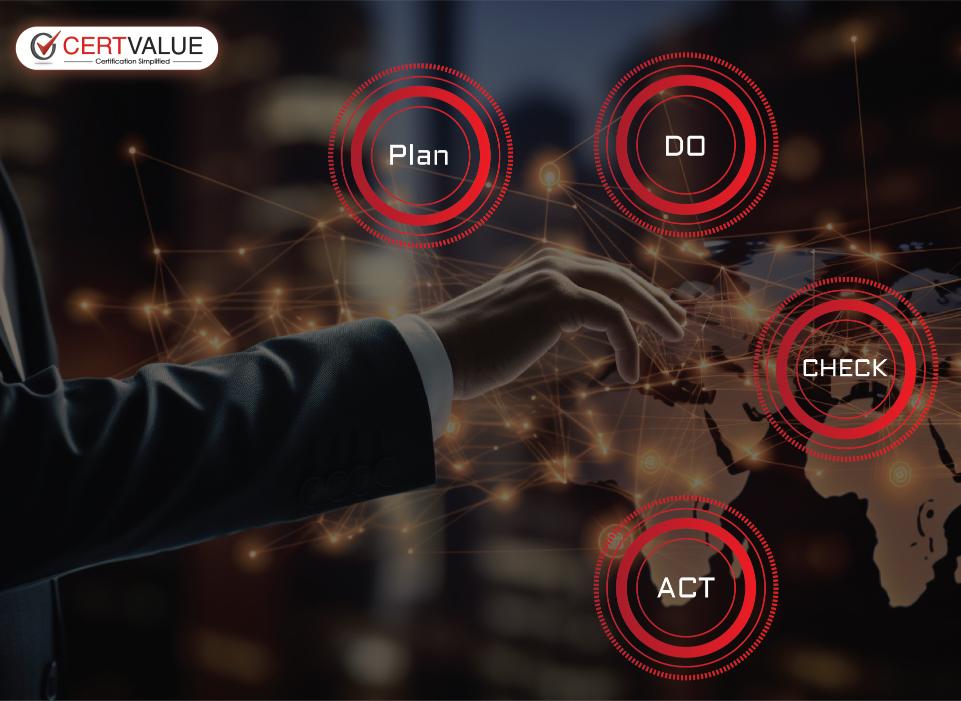
PLAN
Conduct Gap Analysis to find any Shortcomings from the standard requirements.
DO
Policies, procedures, Work Instructions, Evidences, Records, Training
CHECK
Conduct frequent internal audit and management review meeting.
ACT
Apply corrective actions on the identified root cause or shortcomings
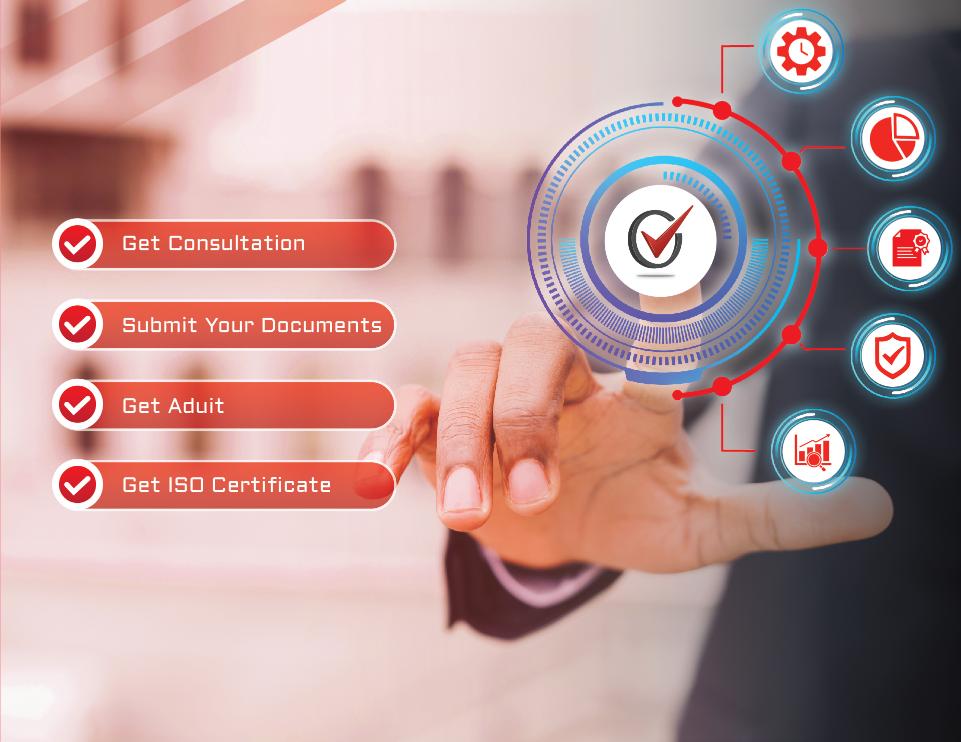
Process for ISO Certification in Bangalore
Certvalue make the ISO certification process in a simple way for every organization in bangalore to get their certification sitting at their place in lesser time and at an affordable cost.
Get Consultation
Conduct Gap Analysis to find any Shortcomings from the standard requirements.
Submit Your Documents
Policies, procedures, Work Instructions, Evidences, Records, Training
Get Audited
Undergo a thorough audit by Certvalue’s auditors to assess compliance.
Get ISO Certificate
Upon successful audit, receive your ISO certification from Certvalue.
WHY CERTVALUE?
CERTVALUE – CREATING VALUE FOR YOUR CERTIFICATION

Client/Compliance
Bottom-line of any business organization is profit and Customers are the only source of Profit. Certvalue will help balancing both customer and compliance requirement at the same time with the help of ISO certification

Enhancement of Performance
ISO certification is a tool to streamline and enhance the process performed internal to the organization. Certvalue indulges in inculcating best industry practices.

Recognition and Brand Value
It is always about the Brand value of your organization in the market and ISO certification from Certvalue can make your organization to be an excel and stand out in the market globally

Tender Eligibility
ISO certification is a basic requirement to bid or participate in any tenders floated by government or private sector. And ISO certification from Certvalue is an assurance win over the tenders.
Extract all the benefits of our quality consultation & implementation
Partner Us to achieve the greatest Accomplishments !!
Our Client Reviews















