Over 3,000+ companies globally trust us
From startups to global enterprise giants, Certvalue empowers to extend the breadth and depth of their customer relationships








Get Instant Quote
Certvalue is one of the best GMP Consultant in Belgium for providing GMP Certification Belgium, Antwerp, Leuven, Brussels, Vilvoorde, Tienen and other major cities in Belgium, with the services of implementation, training, documentation, gap analysis, registration, Audit and templates services at affordable cost to all organizations to get certified under Goods Manufacturing Practices in Belgium. GMP refers to the goods manufacturing practices.

Professional Consulting Company
CONSULTATION & CERTIFICATION
GMP Certification in Belgium is mainly developed for the natural and pharmaceutical product manufacturers. It is a set of guidelines that gives the assurance that your product is safe and correct. It is mainly good manufacturers dedicated to the manufactures and GMP provides assurance for producing safe and quality products according to the Quality standard. GMP in Belgium is responsible for the safety, efficiency and quality of pharmaceutical products and medical devices.
Benefits of GMP Certification in Belgium:
- GMP Certification in Antwerp improves brand value or brand image in the market
- Provide guidelines on how to produce safe and quality products controls.
- Develops customer satisfaction by delivering the safe and quality product and services.
- Develops motivation and teamwork between the employees of the organization.
GMP Certification necessary if there is a quality control laboratory?
Good quality must be manufactured, it cannot be tested into the product afterwards. GMP Certification in Leuven prevents errors that cannot be eliminated through quality control for the finished product. Without GMP Certification it is impossible to be sure that every unit of a medicine is of the same quality as the units of medicine tested in the laboratory
Why is GMP important?
Deprived quality medicines are not only a health safety, but a waste of money for both governments and individual customers.
All guidelines follow a few basic principles:
- Pharmaceutical manufacturing industries must maintain a clean and hygienic manufacturing area.
- GMP Certification in Tienen controlled environmental management System conditions in order to prevent contamination of drug products from other drug or extraneous particulate matter which may render the drug product unsafe for human consumption.
- Manufacturing processes are clearly defined and controlled.
- Manufacturing processes are controlled, and any changes to the process are evaluated. Changes that have an impact on quality of the drug are validated as necessary.
- Instructions and procedures are written by clear and unambiguous language.
- GMP Certification in Mueang Brussels operators are trained to carry out and document.
- Records are manually instruments, during the good manufacture practices that demonstrate that all the steps required by the defined procedures and instructions were in fact taken and that the quantity and quality of expected.
- Records of manufacture that enable the complete history of a batch to be traced are retained in a comprehensible and accessible form.
- The distribution of the drugs minimize any risk to their quality.
- A system is available for recycling any batch of drug from sale or supply chain.
- Complaints about the market, the causes of quality management defects are investigated, and appropriate measures are taken with respect to the defective drugs and to prevent recurrence.
Fundamental rules of GMP to be followed
- Goods Manufacturing Practices in Belgium every instruction should be available in writing before starting that particular task.
- Untrained staff should not carry out a particular task unless there is someone trained available to perform that task.
- Never forget to follow the instructions word by word. No corners should be cut.
- The equipment and materials used for manufacturing must be checked thoroughly and precisely on regular intervals.
- Equipment used must be clean.
- Everything must be kept clean and tidy.
- Keep a check on the mistakes and defects.
- Clear and accurate records of the manufacturing processes must be maintained.
Which industries are eligible to get GMP Certification in Belgium?
Many organizations of varying types Pharmaceuticals Companies, Manufacturing Companies, Food Manufacturing Companies are using GMP as a most recognized method of delivering customer pride and controlling quality of product and Service within their chosen sector GMP not only can be used to supply enhancements and help assurance quality, however the accreditation is often viewed as an assurance of uniformity of product and offerings throughout borders, languages, and cultural boundaries. Therefore, having GMP accreditation can be considered as fine on a reputational basis, as well as a practical one. Implementation of GMP is a different nature of business. So that the Implementation can be done by GMP Consultants for all the industries in Belgium, which improves the customer satisfaction by identifying their needs and goals.
How to get GMP Consultant in Belgium?
How to get GMP Certification in Belgium compliance is widely-accepted as the best way to conduct business, putting quality first. Repressing the original GMP institute, Certvalue GMP courses combine a convenient format with an effective, interactive learning experience. To maximize and customize your professional development. Complete each of the individual Hubli GMP inspection Approach online courses for an overview of all the systems.
Quick Enquiry Form
Our Services
- ISO 9001 Certification
- ISO 14001 Certification
- OHSAS 18001 Certification
- ISO 22000 Certification
- ISO 27001 Certification
- ISO 13485 Certification
- ISO 17025 Certification
- ISO 45001 Certification
- ISO 22301 Certification
- ISO 27701 Certification
- ISO 20000-1 Certification
- ISO 27017 Certification
- ISO 27018 Certification
- ISO 50001 Certification
- ISO 55001 Certification
- ISO 21001 Certification
- ISO 28000 Certification
- ISO 27032 Certification
- ISO 15189 Certification
- ISO 22716 Certification
- ISO 22483 Certification
- CE mark Certification
- HACCP Certification
- HALAL Certification
- SA 8000 Certification
- ROHS Certification
- BIFMA Certification
- GMP Certification

Asked Any Questions
The certificate is issued from a premium certification body and it can be verified from its -website. Every certificate comes with a unique certification number.
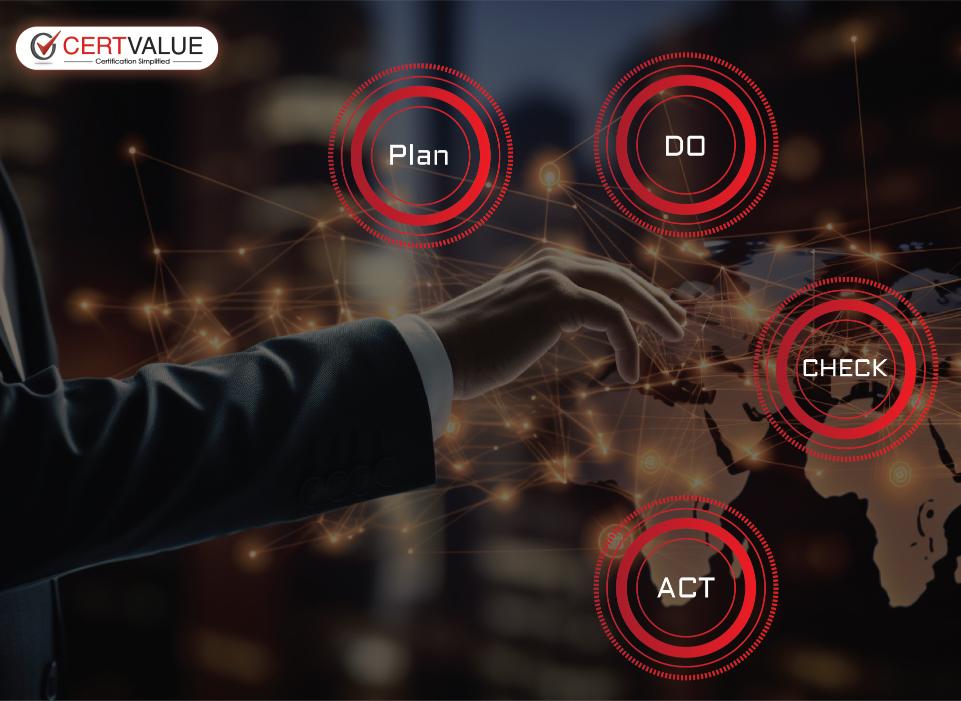
PLAN
Conduct Gap Analysis to find any Shortcomings from the standard requirements.
DO
Policies, procedures, Work Instructions, Evidences, Records, Training
CHECK
Conduct frequent internal audit and management review meeting.
ACT
Apply corrective actions on the identified root cause or shortcomings
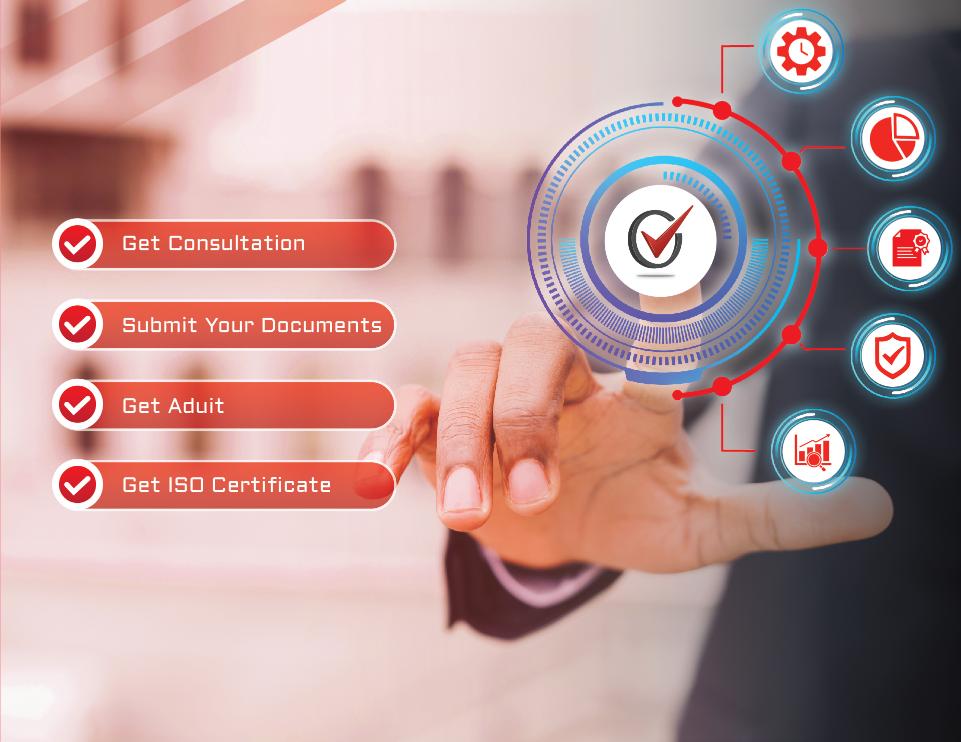
Process for ISO Certification in Bangalore
Certvalue make the ISO certification process in a simple way for every organization in bangalore to get their certification sitting at their place in lesser time and at an affordable cost.
Get Consultation
Conduct Gap Analysis to find any Shortcomings from the standard requirements.
Submit Your Documents
Policies, procedures, Work Instructions, Evidences, Records, Training
Get Audited
Undergo a thorough audit by Certvalue’s auditors to assess compliance.
Get ISO Certificate
Upon successful audit, receive your ISO certification from Certvalue.
WHY CERTVALUE?
CERTVALUE – CREATING VALUE FOR YOUR CERTIFICATION

Client/Compliance
Bottom-line of any business organization is profit and Customers are the only source of Profit. Certvalue will help balancing both customer and compliance requirement at the same time with the help of ISO certification

Enhancement of Performance
ISO certification is a tool to streamline and enhance the process performed internal to the organization. Certvalue indulges in inculcating best industry practices.

Recognition and Brand Value
It is always about the Brand value of your organization in the market and ISO certification from Certvalue can make your organization to be an excel and stand out in the market globally

Tender Eligibility
ISO certification is a basic requirement to bid or participate in any tenders floated by government or private sector. And ISO certification from Certvalue is an assurance win over the tenders.
Extract all the benefits of our quality consultation & implementation
Partner Us to achieve the greatest Accomplishments !!
Our Client Reviews















