Over 3,000+ companies globally trust us
From startups to global enterprise giants, Certvalue empowers to extend the breadth and depth of their customer relationships








Get Instant Quote
Good manufacturing practices (GMP) are the practices required in Algeria in order to conform to the guidelines recommended by agencies that control the authorization and licensing of the manufacture and sale of food and beverages, cosmetics, pharmaceutical products, dietary supplements, and medical devices.

Professional Consulting Company
CONSULTATION & CERTIFICATION
These guidelines provide minimum requirements that a manufacturer in Algeria must meet to assure that their products are consistently high in quality, from batch to batch, for their intended use. The rules that govern each industry may differ significantly; however, the main purpose of GMP is always to prevent harm from occurring to the end user. Additional tenets include ensuring the end product is free from contamination, that it is consistent in its manufacture, that its manufacture has been well documented, that personnel are well trained, and the product has been checked for quality more than just at the end phase. GMP is typically ensured through the effective use of a quality management system (QMS). Looking for the GMP Certification Consultants in Algeria? contact with us.
Why Is important for GMP Consultant Services in Algeria?
The main benefits of obtaining GMP in Algeria are:
- GMP certification in Algeria helps to reduce observations raised on inadequate document practices.
- One of the important benefits by obtaining GMP certification in Algeria is it ensures products are safe for humans.
- GMP certification in Algeria helps prevent and control contamination & cross contamination.
- By obtaining GMP certification in Algeria helps in reducing the risk of mislabeling and adulteration.
- GMP certification in Algeria helps to promotes efficiency and reduces cost of doing business.
What is the process of becoming to be GMP Certification Consultant Services in Algeria?
Good manufacturing practice guidelines provide guidance for manufacturing, testing, and quality assurance in order to ensure that a manufactured product is safe for human consumption or use. Many countries have legislated that manufacturers follow GMP procedures and create their own GMP guidelines that correspond with their legislation.
All guideline follows a few basic principles :
- Manufacturing facilities must maintain a clean and hygienic manufacturing area in Algeria.
- Manufacturing facilities must maintain controlled environmental conditions in order to prevent cross contamination from adulterants and allergens that may render the product unsafe for human consumption or use
- Manufacturing processes must be clearly defined and controlled. All critical processes are validated to ensure consistency and compliance with specifications.
- Manufacturing processes must be controlled, and any changes to the process must be evaluated. Changes that affect the quality of the drug are validated as necessary.
- Instructions and procedures must be written in clear and unambiguous language using good documentation practices
- Operators must be trained to carry out and document procedures.
- Records must be made, manually or electronically, during manufacture that demonstrate that all the steps required by the defined procedures and instructions were in fact taken and that the quantity and quality of the food or drug was as expected. Deviations must be investigated and documented.
- Records of manufacture (including distribution) that enable the complete history of a batch to be traced must be retained in a comprehensible and accessible form.
- Any distribution of products must minimize any risk to their quality.
How to get GMP Certification Consultants in Algeria?
Certvalue is one of the leading GMP Certification Consultants in Algeria to providing GMP certifications we are one of the well recognized firms with authorities in each every industry sector to implement the standard with 100% notoriety of achievement.
Our advice, Go for it!!
If you’re looking how to get GMP Certification Consultants in Algeria you can keep in touch with us at [email protected] or visit our official site at we top GMP Certification Consultants in Algeria, Karnataka, India. Provide your contact details with us, so that one of our certification experts will get in touch with you at the earliest to understand your requirements better and provide best available service at market place.
Quick Enquiry Form
Our Services
- ISO 9001 Certification
- ISO 14001 Certification
- OHSAS 18001 Certification
- ISO 22000 Certification
- ISO 27001 Certification
- ISO 13485 Certification
- ISO 17025 Certification
- ISO 45001 Certification
- ISO 22301 Certification
- ISO 27701 Certification
- ISO 20000-1 Certification
- ISO 27017 Certification
- ISO 27018 Certification
- ISO 50001 Certification
- ISO 55001 Certification
- ISO 21001 Certification
- ISO 28000 Certification
- ISO 27032 Certification
- ISO 15189 Certification
- ISO 22716 Certification
- ISO 22483 Certification
- CE mark Certification
- HACCP Certification
- HALAL Certification
- SA 8000 Certification
- ROHS Certification
- BIFMA Certification
- GMP Certification

Asked Any Questions
The certificate is issued from a premium certification body and it can be verified from its -website. Every certificate comes with a unique certification number.
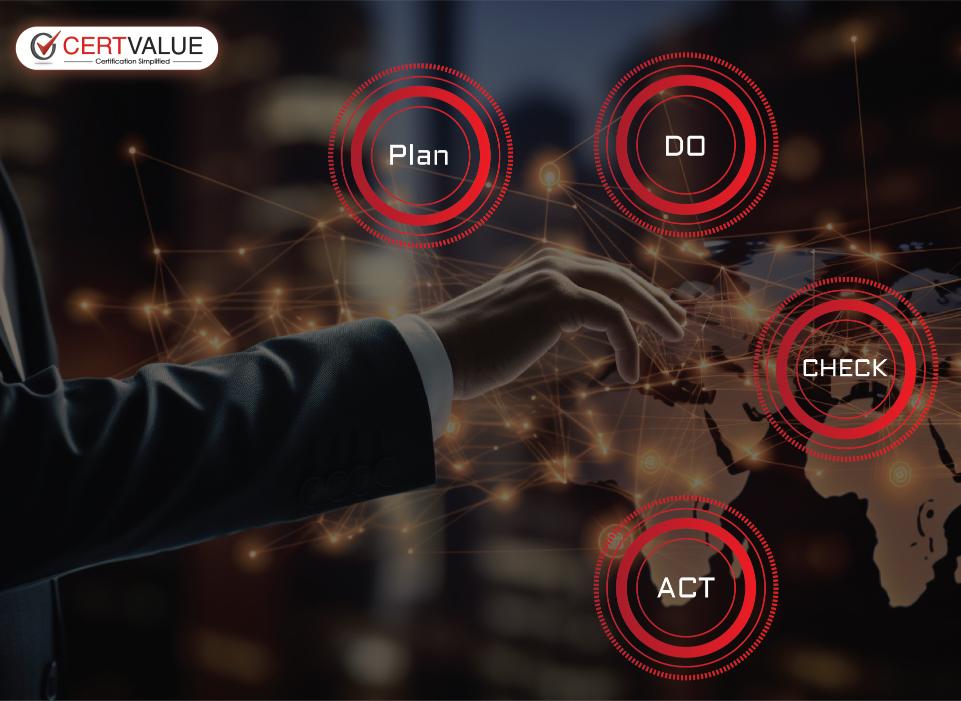
PLAN
Conduct Gap Analysis to find any Shortcomings from the standard requirements.
DO
Policies, procedures, Work Instructions, Evidences, Records, Training
CHECK
Conduct frequent internal audit and management review meeting.
ACT
Apply corrective actions on the identified root cause or shortcomings
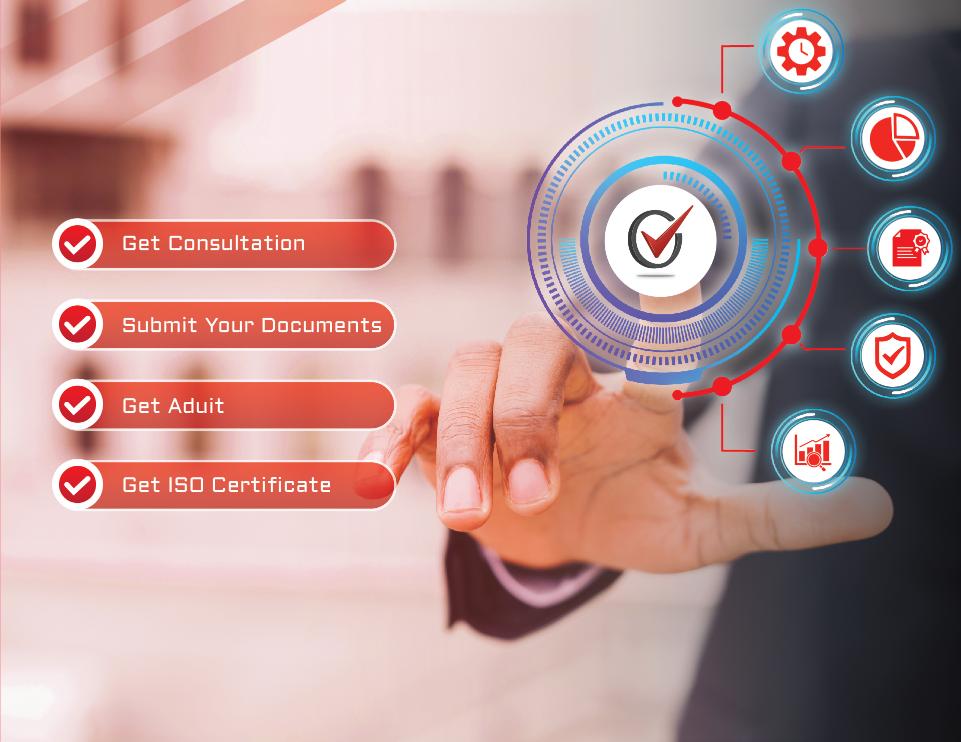
Process for ISO Certification in Bangalore
Certvalue make the ISO certification process in a simple way for every organization in bangalore to get their certification sitting at their place in lesser time and at an affordable cost.
Get Consultation
Conduct Gap Analysis to find any Shortcomings from the standard requirements.
Submit Your Documents
Policies, procedures, Work Instructions, Evidences, Records, Training
Get Audited
Undergo a thorough audit by Certvalue’s auditors to assess compliance.
Get ISO Certificate
Upon successful audit, receive your ISO certification from Certvalue.
WHY CERTVALUE?
CERTVALUE – CREATING VALUE FOR YOUR CERTIFICATION

Client/Compliance
Bottom-line of any business organization is profit and Customers are the only source of Profit. Certvalue will help balancing both customer and compliance requirement at the same time with the help of ISO certification

Enhancement of Performance
ISO certification is a tool to streamline and enhance the process performed internal to the organization. Certvalue indulges in inculcating best industry practices.

Recognition and Brand Value
It is always about the Brand value of your organization in the market and ISO certification from Certvalue can make your organization to be an excel and stand out in the market globally

Tender Eligibility
ISO certification is a basic requirement to bid or participate in any tenders floated by government or private sector. And ISO certification from Certvalue is an assurance win over the tenders.
Extract all the benefits of our quality consultation & implementation
Partner Us to achieve the greatest Accomplishments !!
Our Client Reviews















